Effects and Estimands
An overview of key terminology
Effects
\(Y^{a=1}, Y^{a=0}\) are the potential outcomes under treatment 1 and 0. They are random variables. Treatment A has causal effect if \(Y^{a=1} \neq Y^{a=0}\).
For individual \(i\), \(Y_i^{a=1}, Y_i^{a=0}\) are deterministic.
In reality we do not observe both potential outcomes for an individual, since we only have ONE outcome. We observe \(Y\) and \(A\). For a population, average treatment effect (ATE) can be estimate
Estimands
Greifer, N., & Stuart, E. A. (2021). Choosing the estimand when matching or weighting in observational studies. arXiv preprint arXiv:2106.10577.
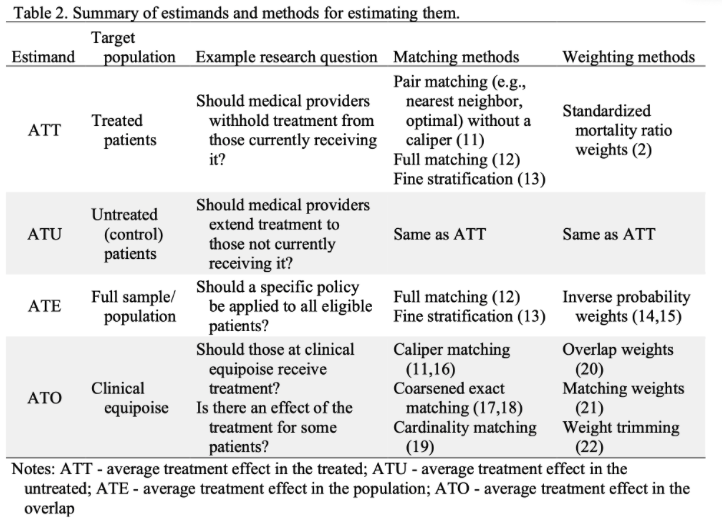
ATE: average treatment effect in the population
\(E[Y(1) - Y(0)]\)
ATT: average treatment effect among the treated
\(E[Y(1) - Y(0) | Z = 1]\)
ATC: average treatment effect among the controls
\(E[Y(1) - Y(0) | Z = 0]\)
ATM: average treatment effect among the matched
Most of the time we care about ATE and ATT. Pay attention to the matching and weight methods:
- ATE commonly uses IPW (inverse probability weighting)
- ATT uses PSM (propensity score matching).
Bias
| Data generation | Correct causal model | Correct causal effect |
|---|---|---|
| Collider | Y ~ X | 1 |
| Confounder | Y ~ X; Z | 0.5 |
| Mediator | Direct effect: Y ~ X; Z. Total effect: Y ~ X | Direct: 0; total: 1 |
| M-Bias | Y ~ X | 1 |
Selection bias
This bias is the result of selecting a common effect of 2 other variables (collider): a treatment, an outcome.
- non-response, missing data
- self-selection, volunteer bias
- selection affected by treatment before study started
A form of lack of exchangeability between the treated and untreated.
Correct for selection bias: IP weighting
ICE (in clinical trial)
Key question: how to present the data visually?
What are considered as important, what are not?
ITT (intention to treat): include the data after rescue medicine. Includes dropouts
PP (Per-protocol): exclude patients taking rescue medicine, only analyse the complete cases
Trial estimands: trial treatment effect depends on how events occur after treatment initiation.
Treatment effect for a given outcome.
Five core attributes
- population
- treatment conditions
- endpoint
- summary measure
- strategies to handle each type of intercurrent event
Intercurrent event
Post-baseline events (post randomisation in RCT) that affects the interpretation of outcome.
Two categories:
treatment-modifying events, affects the receipt of assigned treatment. E.g. early discontinuation, use of rescue, wrong dose, wrong type (placebo for example).
truncating events, e.g. death, amputation of limb when the limb is relevant for the research question.
Strategies (multiple can be used for different ICE in the same study)
- treatment policy strategy: treat as it is, ignore ICE
- composite strategy: modifies the endpoint value, defined by the investigator
- while-on-treatment (while alive): before intercurrent event data is used.
- hypothetical strategy
- principal stratum strategy: redefine the population